 |  |
 |
|
|
Configurational Stability of Chlorophosphines
S. Humbel, C. Bertrand, C. Darcel, C. Bauduin and S. Jugé
Inorg. Chem. 2003, 42, 420-427
|
Chlorophosphines are usefull building blocks for the synthesis of functionalyzed ligands. They would be particularly attractive for the synthesis of chiral ligands that bear the chiral information on the phosphorus atom although they are subject to intantaneous racemization.
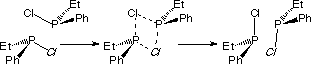 This fact was attributed to a bimolecular process as shown above. In such a mechanism, two chlorophosphines react one on the other and both racemize. This mechanism precludes any possibility to isolate and maintain a chiral phosphorus atom in chlorophosphines.
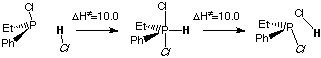 In this work we attributed this racemization to traces of residual chlorhydric acid. We found a mechanism that is much lower in energy (about 10 kcal/mol). Moreover, the mechanism is catalytic in HCl (that is restituted at the end of the reaction). The following picture shows both mecanisms, energetically.
 |
| |
Further readings & informations
|
 |
Omelanczuk, J. J. Chem. Soc. Chem. Commun. 1992, 1718-1719.
Levin, C.C. J. Am. Chem. Soc. 1975, 97, 5649-5655
Bent, H.A. Chem. Rev. 1961, 61, 275-311.
Horner, L.; Jordan, M. Phosphorus and Sulfur 1980, 8, 235-242. |
 |
This subject has been done in collaboration with Prof. Sylvain Jugé, Université de Bourgogne. |
|
| 2006 |
|
|
|