 |  |
 |
|
|
Specific Solvent Effect on R2ZrCl2 (R = Butyl, Ethyl) Reactivity, a Density Functional Study
E. Derat, J. Bouquant, P. Bertus, J. Szymoniak and S. Humbel, J.
Organomet. Chem. 2002, 664, 268-276. |
The formation of zirconacyclopropane starting from di-alkyl zirconium dichloride is computed. Specific consideration of the solvent shows the role of THF and phosphines on the reaction feasibility. 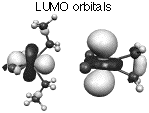
Beta and Gamma migration processes are considered. It is shown that the Beta is much easier than the other.
The Molecular Orbitals of the model system are briefly analyzed to explain the large stabilization of the reaction product (zirconacyclopropane) by the lone pairs of the solvant or co-ligands.
|
| |
Further readings & informations
|
 |
This work was done in a collaboration with Prs J. Bouquant and J. Szymoniak, UMR 6519, Reims. |
 |
Prof Eisch web page
J.J. Eisch, F.A. Owuor, P.O. Otieno, Organometallics 2001, 20, 4132-4134. J.J. Eisch, F.A. Owuor, X. Shi, Organometallics 1999, 18, 1583-1585. J.J. Eisch, X. Shi, F.A. Owuor, Organometallics 1998, 17, 5219-5221. |
|
| 2006 |
|
|
|