 |  |
 |
|
|
Short Strong Hydrogen Bonds: A Valence Bond Analysis
Stéphane Humbel, J.
Phys. Chem. A2002, 106, 5517-5520. |
Short Strong Hydrogen Bond (SSHB) are also called Low Barrier Hydrogen Bond (LBHB). They are about the largest hydrogen bonds known, and are encountered in dimers or in biological systems. The SSHB bond strength is a long standing subject and we present here a new approach to explain empirical formulas that relate SSHB bond strength to the difference in proton affinity of the two basis.
We modeled Short Strong Hydrogen Bonds with two resonating bonding structures issued from a Valence Bond analysis. A formula (equ. 1) giving the bond dissociation energy as a function of the difference in Proton Affinities (ΔPA) is demonstrated. This equation is expanded in Taylor series and compared to similar equations found in the literature. It is found that the correlations either from experimental datas or derived from the Marcus equation can be justified by the same Valence Bond arguments.
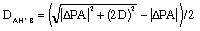  (1) (1)
For the (unsymetrical AH+B system, the D factor is the average of the bond dissociation energies of the corresponding dimers (AH+A and BH+B).
The demonstration is based on a Valence Bond analysis were the 6 possible VB structures (I-VI) are replaced by only 2 resonating bonding structures. (VI is neglected). Such a 2x2 determinant is analytically resolved and Equ. 1 is obtained.
Using Taylor series for small ΔPA, Equ. 1 is related to previous work found is the litterature. 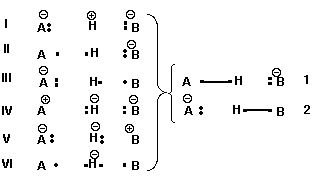
|
| |
Further readings & informations
|
 |
Gilli, G; Gilli, P. J. Mol. Struct., 2000, 552, 1-15. Meot-Ner, M. J. Am. Chem. Soc. 1984, 106, 1257-1264.
Larson, J.W.; McMahon, T.B. J. Am. Chem. Soc. 1982, 104, 6255-6261. a) Zeegers-Huyskens, T Chem. Phys. Lett. 1986, 129, 172-175; b) Zeegers-Huyskens, T J. Mol. Struct. 1988, 177, 125-141. Scheiner, S. J. Am. Chem. Soc. 1981, 103, 315‚320. a) Scheiner, S.; Redfern, P. J. Phys. Chem. 1986, 90, 2969-74; b) Magnoli, D.E.; Murdoch, J.R. J. Am. Chem. Soc. 1981, 103, 7465-7469 |
 |
Related web sites Prof. Steve Scheiner Prof. Gastone Gilli |
|
| 2006 | | | |