 |  |
 |
|
|
Lewis-Based Valence Bond Scheme:
Application to the Allyl Cation
Mathieu Linares, Benoît Braïda, Stéphane Humbel, J.
Phys. Chem. A 2006, 110, 2505-2509. |
The allyl cation can be written as resonance between 2 or even 3 Lewis structures (I II III). Although it looks rather odd, the 3rd structure (III) was found (by Y. Mo in JCP 1996) to weight about 20% in the total wave function.
Quantum chemistry methods normally cannot use such a simple view of the resonance, even in such a simple system. For instance in the traditionnal Valence Bond (VB) formalism the equivalent description would require a much larger number of structure (from a to i although some redondancies push some VB structures to fade away as tentatively represented bellow).
A method for expressing the wave function in the simple terms of Lewis structures is proposed and tested on the allyl cation. 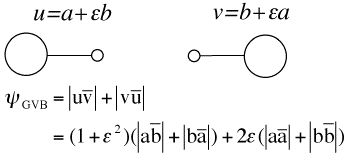 This computational scheme is called Valence Bond BOND for Breathing Orbitals Naturally Delocalized, and labelled as VBB. It makes use of a GVB description of each double bond, which ensures that left-right correlation is accounted in each double bond. This computational scheme is called Valence Bond BOND for Breathing Orbitals Naturally Delocalized, and labelled as VBB. It makes use of a GVB description of each double bond, which ensures that left-right correlation is accounted in each double bond.
The compact VBB wave function gives consistent results with the Breathing Orbital Valence Bond method (BOVB from Hiberty et al) for the resonance energy of the allyl cation (54 and 55 kcal/mol for VBB and BOVB, respectively). The optimization of the σ orbitals, in such a way they adapt to each resonance structure, makes use of the Breathing Orbital Effect. It is shown that this "breathing" of the σ frame is more efficient in the resonant hybrid than in the localized state, so that a resonance energy of 63 kcal/mol is obtained at this level of computation.
The weights found with the VBB method is consistent with the NRT computation results.
|
| |
Further readings & informations
|
 |
Mo, Y.; Lin, Z.; Wu, W.; Zhang, Q. J. Phys. Chem. 1996, 100, 11569-11572 |
 |
Hiberty, P.C.; Flament, J.P.; Noizet, E. Chem. Phys. Lett. 1992, 189, 259-265. Hiberty, P.C.; Humbel, S.; Archirel, P. J. Phys. Chem. 1994, 98, 11697-11704
Hiberty, P.C.; Humbel, S.; Byrman, C.; van Lenthe, J. H. J. Chem. Phys. 1994, 101, 5969-5976. (d) Hiberty, P.C. J. Mol. Struct. (Theochem) 1998, 451, 237-261. |
 |
Review on NBO & NRT Methods and their Applications: (a) A. E. Reed, L. A. Curtiss and F. Weinhold Chem. Rev. 1988, 88, 899-926. (b) Weinhold, F.; Carpenter, J.E. in The Structure of Small Molecules and Ions; Naaman, R.; Vager, Z.; Eds.; , Plenum: New York, NY 1988
See also the web page of NBO 5.0 that we used with Gaussian 98. NBO 5.0
and the Huckel code for mesomery calculations+ the light version Huckel for iphone
|
|
| 2006 | | | |