 |  |
 |
|
|
Reactivity of Dialkylzirconium Species and Solvent Polarity
E. Derat, J. Bouquant, P. Bertus, J. Szymoniak, S. Humbel Int. J. Quant. Chem.2006, 106, 704-711 |
The reactivity of zirconium related species is studied to explain a strong solvent effect. The whole description starts from the di-alkyl zirconium dichloride species. Specific consideration of the solvent shows why ansa-species are formed in polar solvent while the hydrogenation product is obtained in apolar solvent.
Molecular Orbitals' study of a model system are briefly analyzed 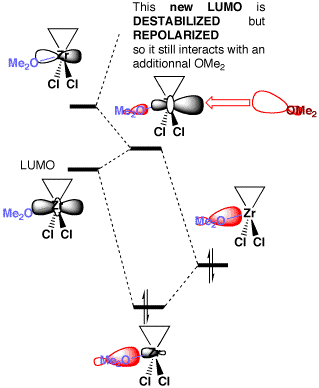 to explain the large stabilization of the reaction intermediate (zirconacyclopropane) by the lone pairs of two OMe2 moieties. to explain the large stabilization of the reaction intermediate (zirconacyclopropane) by the lone pairs of two OMe2 moieties. This illustrates the DESTABILIZATION and the REPOLARIZATION of the LUMO by the lone pair of the first OMe 2 "Ligand" so the second one still interacts quite efficiently with the new LUMO.
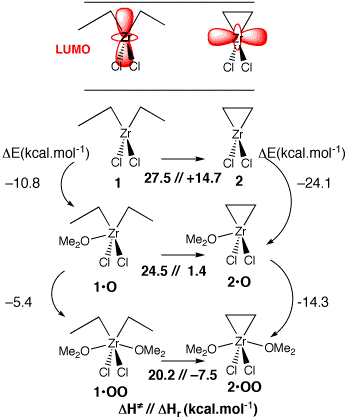 The stabilization energies are smaller for the additionnal ligand (from –24.1 to –14.3 kcal.mol-1) but the key is that the zirconacyclopropane formation is exothermic if OMe2 ligands are present, while it is endothermic in gas phase (which is assimilated to apolar solvant).
The selectivity is explained from this endo- / exo- thermicity of the first reaction.
|
| |
Further readings & informations
|
 |
See also the other papers on the subject:
Specific Solvent Effect on R2ZrCl2 (R = Butyl, Ethyl) Reactivity, a Density Functional Study E. Derat, J. Bouquant, P. Bertus, J. Szymoniak and S. Humbel, J. Organomet. Chem. 2002, 664, 268-276.
Hydrometalation or Condensation in the Reaction of Cl2ZrEt2 with H2CO. A Theoretical Account. E. Derat, J. Bouquant, P. Bertus, J. Szymoniak and S. Humbel, Organometallics 2004, 23, 2892-2899. |
 |
This work was done in a collaboration with Prs J. Bouquant and J. Szymoniak, UMR 6519, Reims. |
 |
Prof Eisch web page
J.J. Eisch, F.A. Owuor, P.O. Otieno, Organometallics 2001, 20, 4132-4134. J.J. Eisch, F.A. Owuor, X. Shi, Organometallics 1999, 18, 1583-1585. J.J. Eisch, X. Shi, F.A. Owuor, Organometallics 1998, 17, 5219-5221. |
|
| 2006 |
|
|
|